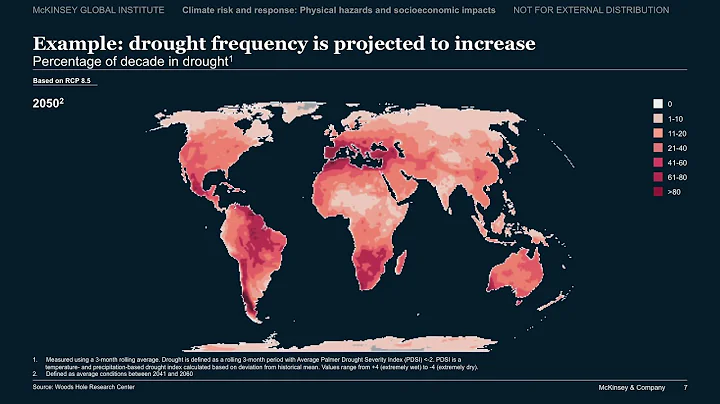
In June 2022, the Canadian government released a draft risk assessment framework for man-made nanoparticles in accordance with the Canadian Environmental Protection Act (CEPA). The comment collection period started on June 17 and ended in 60 days.
Nanotechnology is defined as a method of processing materials with nanometer scales between 1-100nm. Artificial nanomaterials (NMs) developed using this technology are rapidly entering the Canadian market across a wide range of applications and industries. Chemical substances, including nanomaterials, are regulated under the Canadian Environmental Protection Act (CEPA), which authorizes the collection of information, assessment and management of risks to the environment and human health. The principles of chemical evaluation are applicable to the evaluation of nanomaterials, with necessary modifications to address the specificities of nanomaterials.

Based on the risk assessment of nanomaterials under CEPA:
- The risk assessment of NMs follows the principles of chemical risk assessment, including application and precautionary measures based on the weight of evidence (WoE);
- In the absence of international consensus on the nanonomenclature system, the use of chemical Abstract Service Register Number (CAS RN2) and physicochemical property information (e.g., size, shape, surface chemistry) to identify and characterize the range of nanoscale forms;
- is often required to assess the size distribution, shape, and surface chemistry of nanoscale produced substances ;
- A substance is assessed as nano if 10% or more (by number) of its primary particles have at least one internal or external dimension, or are on the scale of 1 to 100 nanometers. If a particle size distribution quantity is not available, a substance is assessed as nanoscale if at least 1% (by mass) of the primary particles are at the nanoscale or have at least one internal or external dimension within the nanoscale;
- cross-reference for nano The law is under development. At this time, cross-referencing to nano is considered on a case-by-case basis and as part of the WoE approach risk assessment. Cross-reference law guidance on Nano provided by other jurisdictions/organizations (e.g. European Union , OECD) was also considered. Grouping strategies for nanoecological and human health risk assessment may differ.
- The conclusions drawn from the risk assessment may apply to a range of known nanovariants of the CAS RN assessed, or different conclusions may be drawn for different nanoforms with the same CAS RN;
- The conclusions under Article 64 of CEPA are inconsistent with the Dangerous Products Regulation The Hazardous Materials Information System for Workplace Hazardous Materials Information System is not relevant to, and does not preclude the evaluation of, hazard standards for products intended for use in the workplace.
Final Screening Assessment
The Canadian government has also formally concluded that sucrose acetate isobutyrate (SAIB) and p-toluenesulfonic acid (PTSA) meet the standards set out in paragraph 64(c) of CEPA. Because it enters the environment in quantities or concentrations that do not pose or may pose a risk to human life or health. The government does not intend to take any regulatory action on these substances.
Source: Huaguitong