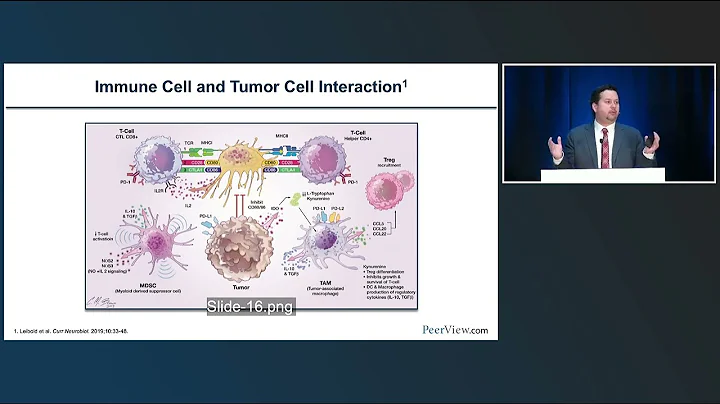
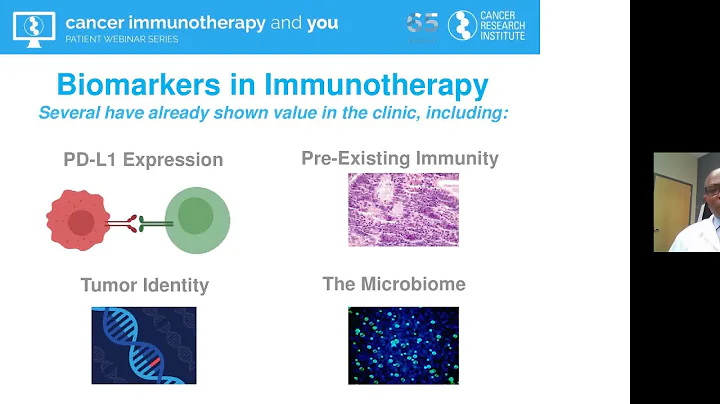
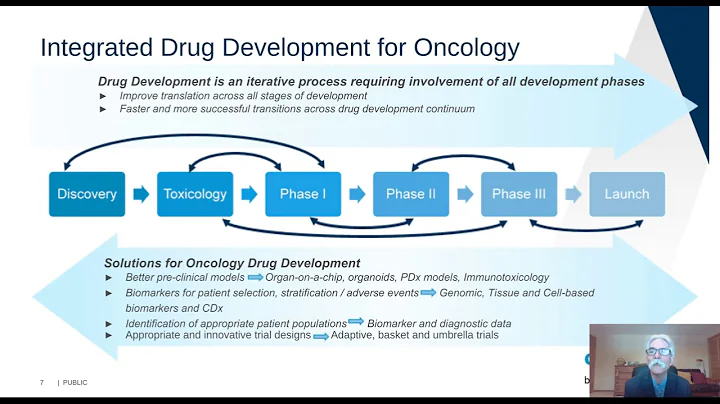
Biomarker 's development and transformation research play a very important role in the research and development of new drugs. The biomarkers of immunotherapy are different from targeted therapies and are relatively complex. It is often impossible to use a single drug target marker to treat patients. Stratification, efficacy prediction. Currently, the FDA has approved immunotherapy biomarkers PD-L1, MSI, TMB, etc. for companion diagnosis, but these markers still have certain limitations.
When PD-L1, TMB, and MSI are used as biomarkers alone, they can distinguish benefit groups to a certain extent; however, they all have high false positive and certain false negative rates, and the prediction of immunotherapy efficacy is not accurate enough; at the same time, in combination with immune It has no predictive value for efficacy in treatment. The road to exploration of IO biomarker is long and arduous.
There are currently two strategies for the conversion of biomarkers in tumor immunotherapy. The first strategy is to verify known IO biomarkers and joint analysis of markers in new therapies; the second strategy is to conduct appropriate biological testing in the early stages of drug development. Marker exploration.
Strategy 1
Verify known IO biomarkers in new therapies, joint analysis of markers
Taking TMB as an example, simply using the mutation number algorithm to characterize immunogenicity is not accurate, and more and more high-level evidence-based Medical evidence suggests that in addition to the "quantity" of mutations, the "quality" of mutations should be considered.

In the research on new immunotherapy biomarkers such as PD-1 combined with chemotherapy, anti-angiogenic drugs, CTLA-4, LAG 3, etc., various research results have demonstrated new biomarkers such as TIS, TIS+TMB, ITH+TMB, etc. Relevant application value in immunotherapy and immune combination therapy of pan-cancer species .



Strategy 2
Exploring appropriate new biomarkers in the early stages of drug development

In December 2021, CDR released the "Technical Guiding Principles for the Application of Biomarkers in the Clinical Research and Development of Anti-tumor Drugs", which clearly pointed out the role of biomarkers in the development of new anti-tumor drugs. The value of biomarkers has become increasingly prominent and has become an extremely important component in the research and development of anti-tumor drugs, encouraging earlier and more planned exploration and research of biomarkers.
In 2017, Yuce Biotech and Academician Zhan Qimin initiated the anti-cancer "Ladder Plan" database based on cooperation, mutual benefit and win-win, aiming to accumulate IO treatment cohort data and accelerate the clinical transformation of tumor immunotherapy. currently cooperates with nearly 50 IO clinical studies and has accumulated nearly 5,000 cases. Based on the Tianti Project data platform, Yuce Biotech has developed a number of tumor immune biomarker algorithms with independent intellectual property rights.
The complexity of the tumor microenvironment determines that tumor immunotherapy biomarkers need more exploration, especially based on new technologies such as single-cell sequencing and spatial transcriptome, plasma proteomics, artificial intelligence (AI). Starting in 2020, Yuce Biotech has successively introduced internationally leading immune microenvironment research platforms based on the high-throughput sequencing platform, including gene expression profiling, histopathology, spatial transcriptomics, and plasma proteomics. and other technology platforms, and through practical summary, a complete set of biomarker translation research solutions has been formed.

Gene Expression Profile marker development is a very important direction. Tumor tissue RNA expression can provide a more comprehensive exploratory analysis of the tumor microenvironment. RNA sequencing or nCounter IO360 can be performed on retrospective samples from early clinical trials. Panel testing and bioinformatics methods to develop relevant gene expression profiles (RNA signatures) can more sensitively and specifically screen people who will benefit from immunotherapy.



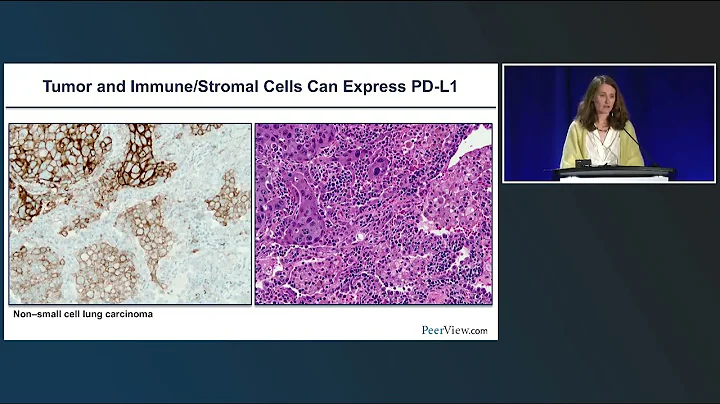
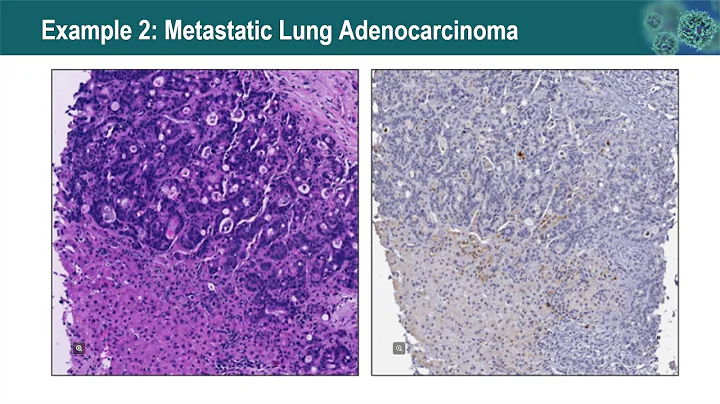
Compared with PD-L1 (IHC), TMB, and GEP, mIHC (mIF) seems to be more effective in evaluating the efficacy of immunotherapy, perhaps because proteins as functional molecules can more accurately reflect the status of the tumor microenvironment. At the same time, tumor immunity The response mechanism is complex, and the expression of a single protein cannot accurately reflect the tumor immune response.

Therefore, Yuce built a pathology platform. The main experimental process of mIF includes staining - scanning - image analysis. It is equipped with a Leica bond RX automatic staining machine to ensure the stability of multiple rounds of multi-color immunofluorescence staining. Akoya Polaris Scanning system to improve image quality. Image analysis introduces HALO, a pathological image analysis tool for mIHC/IHC/HE. It is highly recognized in the drug R&D and clinical research industries and can achieve rich functions. It is a widely used tool in quantitative analysis of pathological images. The combination of halo and inform can realize spatial distance analysis, TILs infiltration analysis, third-level lymphoid structure TLS analysis, tumor tissue immunophenotyping analysis, etc.

There is no shortage of excellent drugs in current drug discovery, but many drugs have not been screened for suitable treatment groups. Researchers should explore multi-omics biomarkers earlier and in a planned way, and develop companion diagnostic products to improve clinical experiments. Success rate.