
Immune checkpoint inhibitors have changed the treatment guidelines for advanced malignant solid tumors. However, the evidence for the use of immune checkpoint inhibitors in neoadjuvant therapy is insufficient. Recently, a phase 1b clinical study led by Academician He Jie of the Cancer Hospital of the Chinese Academy of Medical Sciences was published in JTO magazine, providing new evidence for neoadjuvant immunotherapy for non-small cell lung cancer (NSCLC).
To this end, Professor Qin Haifeng, the founder of i Hope College, specially invited Professor Xu Song from Tianjin Medical University General Hospital to share his analysis and thoughts on the latest developments.
Background:
Chemotherapy, radiotherapy and surgical resection are the traditional treatments for NSCLC. For patients with stage II or above, adjuvant radiotherapy and chemotherapy are usually performed after surgical resection to achieve better therapeutic effects. Although adjuvant therapy has improved survival rates, the 5-year survival rate of NSCLC is still unsatisfactory. Neoadjuvant immunotherapy for NSCLC patients can activate T lymphocytes to attack tumors and prolong survival time as much as possible. In addition, neoadjuvant treatment response can guide subsequent adjuvant treatment selection. Previous studies have shown that NSCLC patients can benefit more from neoadjuvant therapy compared with conventional therapy. However, many patients who receive neoadjuvant chemotherapy often experience adverse events, such as fatigue, pneumonia, and hypothyroidism, which limits Application of new adjuvant chemotherapy . Therefore, as an emerging treatment method with better clinical results and fewer complications, immunotherapy is regarded as the main treatment method for preoperative treatment of various cancers (including NSCLC).
In this study, researchers followed patients with NSCLC for 3 years and revealed the safety and efficacy of neoadjuvant sintilimab. In addition, the efficacy of subgroups of patients (which may be used as predictive factors: PD-L1 expression, tumor mutation burden, major pathological response) was also evaluated.
Method:
- Sintilimab (200 mg, intravenous injection) is used for patients with stage IA-IIIB NSCLC (registration number: ChiCTR-OIC-17013726). Surgery is performed within 29 to 43 days after treatment. All patients underwent PET/CT at enrollment and before surgery to evaluate tumor metabolism after PD-1 inhibitor treatment. At the same time, the researchers conducted an exploratory analysis of the expression of PD-L1 in 32 eligible patients (Figure 1). Safety is the primary endpoint. Key secondary endpoints include overall survival (OS), disease-free survival (DFS), event-free survival and major pathological response.
Deputy Chief Physician and Associate Professor of Lung Cancer Surgery, Tianjin Medical University General Hospital,
Master’s Tutor
Deputy Director and Party Branch Secretary of Tianjin Lung Cancer Research Institute
Tianjin Lung Cancer Metastasis and Tumor Microbiome environment Deputy Director of Key Laboratory
M.D., Free University of Brussels, Belgium
AATS Thoracic Surgery Training Fellowship recipient
ASCO Virtual Mentoring Program winner•Mayo Clinic, MGH, UCM and TGH Clinical Visiting Scholar
Tianjin Distinguished Professor Young Scholar
Tianjin University "Middle School" Young Backbone Innovative Talents"
Tianjin's high-level talents in the health and family planning industry"Young Medical Emerging Talents"
Tianjin's Innovative Talent Promotion Plan "Young Science and Technology Outstanding Talents"
Tianjin's "131 Innovative Talent Project" second-level candidates • CSCO "35 Most Potential Oncology Doctors Under 35" /CSCO "35 under 35" Growth Star Award
Tianjin Medical University General Hospital "Top Ten Doctors"
First Prize of Tianjin Science and Technology Progress Award in 2020
2020 China Medical Technology Award Medical Science and Technology Award Third Prize
2015 China Anti-Cancer Association Science and Technology Award Third Prize
Tianjin Medical University General Hospital
2020 Graduate Student of Lung Tumor Surgery
took the first place/common The first author published in SCI 2 papers
- . Mariano Provencio-Pulla. et al. Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. https: //meetings.asco.org/abstracts-presentations/208906 . Mariano Provencio-Pulla. et al. Neoadjuvant nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) versus chemo for resectable (IB-IIIA) non-small cell lung cancer (NSCLC): Association of pathological regression with event-free survival (EFS) in CheckMate 816. https://meetings.asco.org/abstracts-presentations/208905

Figure 1 Research flow chart
Results
Clinical characteristics of patients
Between March 6, 2018 and March 8, 2019, a total of 43 NSCLC patients were screened, and 40 patients were finally included. Among the 40 enrolled patients, 32 (80%) had a history of smoking, 33 (82.5%) had squamous cell carcinoma , 8 (20%) had stages IA and IB, and 14 (30%) had squamous cell carcinoma . ) were stage IIA and IIB, 10 cases (25%) were stage IIIA, and 8 cases (20%) were stage IIIB (eighth edition of TNM staging). PD-L1 expression was evaluated in 32 patients (80%), of which 22 (55%) had positive PD-L1 expression; 37 (92.5%) patients underwent surgery after receiving neoadjuvant therapy (2 courses of sintilimab) treat. Among them, 36 cases were R0 resection (Figure 2), and 1 case was R2 resection (mediastinal lymph node invasion).

Figure 2 Clinical information of patients
Recurrence situation
A total of 8 cases of recurrence (22.2%), including 1 case of local recurrence, 3 cases of lung metastasis, 3 cases of brain metastasis, and 1 case of bone metastasis. Among these 8 patients, 1 case (12.5%) had recurrence within 1 year after operation, and 6 cases (75.0%) had recurrence within 2 years after operation.
survival time
average follow-up time was 37.8 months (1.73~46.5 months). The researchers analyzed the survival time of two cohorts of patients: patients with R0 resection (36 patients) and patients with positive PD-L1 expression (TPS ≥ 1%) (22 patients). Among the 36 patients with R0 resection, the 3-year OS rate was 88.5% and the 3-year DFS rate was 75.0% (Figure 3A). In the PD-L1-positive cohort, the 3-year OS rate was 95.5% and the 3-year DFS rate was 81.8% (Figure 3B).

Figure 3 Survival analysis
Patients were divided into high, intermediate, and low PD-L1 subgroups (1%, 10%, and 50%) based on PD-L1 expression levels. DFS analysis showed that compared with patients with PD-L1<1%,>
Figure 4 Subgroup analysis
Safety
Adverse reactions (AEs) and neoadjuvant therapy-related AEs (TRAEs) were observed in 32 and 21 patients, respectively. Two patients died within a short period of time after surgery, and the cause of death was severe AE: 1 patient died of disturbance of consciousness on the 8th day after surgery; 1 patient died of immune-related pneumonia after surgery.
Conclusion:
Based on 3 years of follow-up, this study provides the first long-term, comprehensive survival data on the neoadjuvant treatment of PD-1 inhibitors in NSCLC patients, proving the effectiveness and feasibility of neoadjuvant sintilimab. , especially for patients with positive PD-L1 expression.
i Hope time
i Hope College founder
Qin Haifeng Professor
i Hope College founder Professor Qin Haifeng specially invited Professor Xu Song from Tianjin Medical University General Hospital to share his analysis and thoughts on the latest developments.
Introduction to the expert team

Professor Xu Song

Zhou Ning
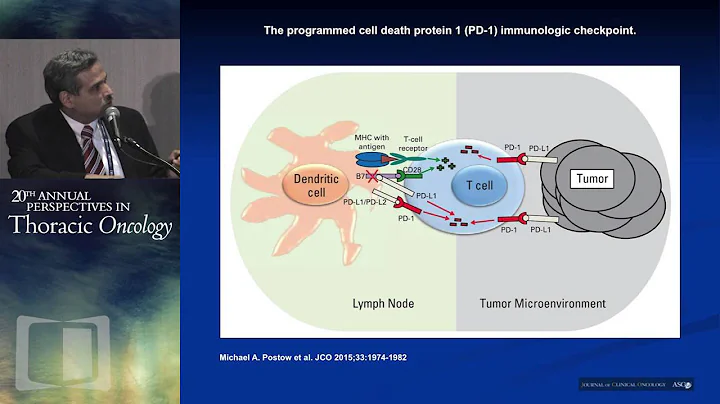
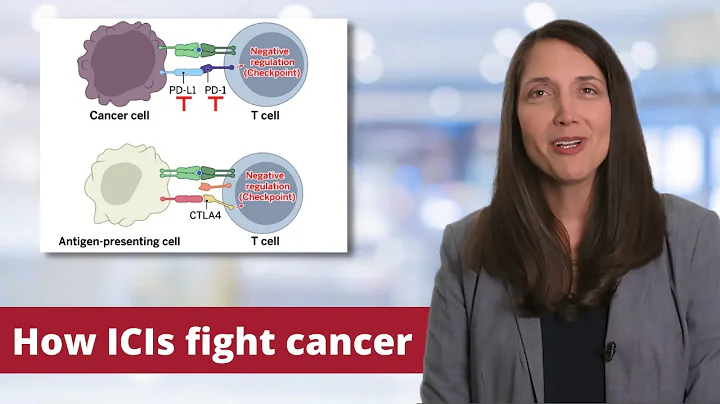
Professor Xu Song : In recent years, immune checkpoint inhibitors represented by PD-1/PD-L1 have made great progress in the treatment of non-small cell lung cancer (NSCLC) and are gradually used in NSCLC. neoadjuvant treatment. Sintilimab is a PD-1 inhibitor independently developed in my country and has shown good efficacy in multiple cancer types. In this study initiated by Academician He Jie and Professor Gao Shugeng, neoadjuvant immunotherapy for NSCLC achieved great success.
A total of 40 patients with stage IA-IIIB NSCLC were included in this study, with an average follow-up time of 37.8 months. The 3-year overall survival (OS) reached 88.5%, the 3-year disease-free survival (DFS) rate reached 75%, and the overall recurrence rate was 22.2%. Further subgroup analysis showed that patients with positive PD-L1 expression (≧1%) were more likely to benefit from neoadjuvant immunotherapy, with their 3-year OS and DFS rates reaching 95.5% and 81.8% respectively. Neoadjuvant immunotherapy showed longer OS compared with neoadjuvant chemotherapy. Additionally, this study explores biomarkers for neoadjuvant immunotherapy. The study showed that although there was no statistically significant difference, positive PD-L1 expression (≧1%), high tumor mutation load (TMB≧10) and better pathological response rates (MPR and pCR) still showed better Good immunotherapy benefit trends.
In the current clinical trials of neoadjuvant immunotherapy for NSCLC, many studies have shown that neoadjuvant immunotherapy is more effective than neoadjuvant chemotherapy.At this year's ASCO meeting, a study by Samuel Rosner et al. (NCT02259621) confirmed the effectiveness of neoadjuvant immunotherapy with Nivolumab.2 Its 3-year, 4-year and 5-year survival rates reached 85%, 80% and 80% respectively; its 3-year, 4-year and 5-year disease-free survival rates also reached 65%, 60% and 60%. The study by Provencio M et al. (NADIM) shows that the effect of neoadjuvant therapy with immunity + chemotherapy is significantly better than that of neoadjuvant chemotherapy. Its 3-year OS and PFS achieved 81.9% and 69% respectively. In addition, in the CheckMate816 study, the median EFS of the neoadjuvant immune + chemotherapy group was significantly longer than that of the neoadjuvant chemotherapy group, which were 31.6 months and 20.8 months respectively. Therefore, neoadjuvant immunotherapy (+/-chemotherapy) is feasible in NSCLC and its effect is better than neoadjuvant chemotherapy.
Although neoadjuvant therapy has been shown to be effective, biomarkers that are sufficiently reliable to predict efficacy are still lacking. Research by Samuel Rosner et al. found that positive expression of MPR and PD-L1 was associated with longer recurrence-free survival.2. In the NADIM study, acquisition of MPR and ctDNA clearance in primary tumors was also confirmed to be associated with longer DFS3. The CheckMate816 study also confirmed that MPR can improve patients' EFS4. Therefore, the expression of PD1/PD-L1, TMB, MPR/pCR, and blood ctDNA levels may be advanced biomarkers for neoadjuvant immunotherapy, but their sensitivity and specificity still require further study.
Current clinical studies mainly use OS or DFS as the research endpoint, but in neoadjuvant immunotherapy trials, MPR/pCR is mainly used as the research endpoint. MPR/pCR has been proven to predict DFS, RFS, and EFS, but its correlation with OS needs further confirmation. In future clinical trials, whether MPR/pCR can replace OS as a new reliable research endpoint still requires more research to confirm.
There are still a number of NSCLC neoadjuvant immunotherapy clinical trials ongoing, and OS and DFS data are still incomplete. Therefore, we also look forward to more studies to confirm the OS benefit of neoadjuvant immunotherapy and bring more treatment options to patients with lung cancer.
References
 . Zhang F, Guo W, Zhou B, et al. Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2022;S1556-0864(22) 00216-7. doi:10.1016/j.jtho.2022.04.012
. Zhang F, Guo W, Zhou B, et al. Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2022;S1556-0864(22) 00216-7. doi:10.1016/j.jtho.2022.04.012- . Samuel Rosner. et al. Neoadjuvant nivolumab in early-stage non–small cell lung cancer (NSCLC): Five-year outcomes. https://meetings.asco .org/abstracts-presentations/208907
Editor: XY
Typesetting: XY
Execution: XY













![Rethinking Physics Informed Neural Networks [NeurIPS'21] - DayDayNews](https://i.ytimg.com/vi/qYmkUXH7TCY/hq720.jpg?sqp=-oaymwEcCNAFEJQDSFXyq4qpAw4IARUAAIhCGAFwAcABBg==&rs=AOn4CLDq7F6iLHKhjmAFymJhtlcYwaXiMw)
![AI/ML+Physics Part 1: Choosing what to model [Physics Informed Machine Learning] - DayDayNews](https://i.ytimg.com/vi/ARMk955pGbg/hq720.jpg?sqp=-oaymwEcCNAFEJQDSFXyq4qpAw4IARUAAIhCGAFwAcABBg==&rs=AOn4CLBMVhxazCgh0pAm1KCjDm3G5IBQXw)



